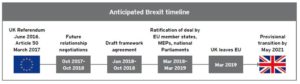
The UK is expected to exit the European Union from 29 March 2019. This has consequences for UK life sciences operations. Have you planned your response?
Life sciences companies trading in both the European Union and the UK will face changes post-Brexit:
- A UK-held medicinal product Marketing Authorization may not be mutually recognized in other EU countries
- A hard border between the UK and EU will present cross-border trade challenges
- Quality Release requirements may change
Navigating the changes requires diligence and coordination. Lead times involved in getting ready to operate in a post-Brexit environment mean that companies need to take action now to secure business continuity, regulatory compliance and market access in both the UK and EU.
Key implications
Marketing and authorization holder:
- EU Marketing Authorizations must be transferred from the UK to an EU-based company
- The UK will require a separate license recognized by Medicines and Healthcare products Regulatory Agency (MHRA)
Quality release:
- Quality-related operations and systems must be relocated to be performed in the EU:
- Qualified Person Responsible for Pharmacovigilance
- Pharmacovigilance System Master File
- Sites of batch release and authorized batch control
- The UK will require separate MHRA-recognized quality elements
Supply chain physical and legal flows
Many organizations have struggled with disjointed Brexit planning. EY’s integrated approach accounts for all Brexit impacts, through a dynamic plan that brings together all relevant business functions:
Diagnose: Conduct scenario analysis to identify the client-specific exposures and opportunities arising from Brexit
Plan: Develop a dynamic, scenario-based plan to respond to any Brexit outcome, detailing no-regrets responses and risk mitigations
Execute: Deliver the relevant changes, to ensure your business is ready for post-Brexit operations
Benefits of our approach
- We have the UK/EU political connections and internal cross-functional expertise to interpret the latest directives, and ensure that client requirements are accurately scoped — not overstated
- We have structured many life sciences European operating models, establishing the legal, regulatory, financial and supply chain elements
- We apply our Brexit scenario models and our complete functional activity plan with our clients, to aid decision-making and time-critical change-planning
Learn more about our approach to Brexit in our full report, Are you ready for a future outside of the European Union?
Compliments of Ernst & Young, a member of the EACCNY
![European American Chamber of Commerce New York [EACCNY] | Your Partner for Transatlantic Business Resources](https://eaccny.com/wp-content/uploads/2020/06/eaccny-logo.png)